
by Julie Matthews, MS | May 7, 2024 | All Posts, BioIndividual Nutrition Articles
The right bioindividual nutrition plan for your client can bring about profound improvement and benefit. In this article, I want to focus on some diets that have received a lot of attention in the news and nutrition community: the ketogenic diet and intermittent...

by Julie Matthews, MS | Apr 23, 2024 | All Posts, BioIndividual Nutrition Articles
Do you experience random headaches? Or perhaps your tongue gets all swollen when you eat tomatoes?As you’ll discover in this article, these symptoms aren’t ‘all in your head’ but could be related to how your body handles histamine. I’ll also talk about what...

by Julie Matthews, MS | Jan 3, 2024 | BioIndividual Nutrition Articles, Chronic Conditions, Scientific Research
New science and clinical experience reveal concerns about oxalates that far exceed traditional kidney stone pathology. In order to best support their patients and clients, integrative practitioners, and especially diet and nutrition specialists would benefit from...

by Julie Matthews, MS | Jan 3, 2022 | All Posts, BioIndividual Nutrition Articles
Gastrointestinal Disorders Prevalence If you’re a nutrition or health practitioner, you’ve certainly had your fair share of clients with a gastrointestinal disorder or symptoms. I’m certain of that. That’s because digestive disorders are incredibly common. They...

by Julie Matthews, MS | Oct 28, 2021 | All Posts, BioIndividual Nutrition Articles
How Our Genes vs. Microbiome Affect Blood Sugar and Foods We Tolerate The primary goal of personalized nutrition is to create a diet that best meets the needs of an individual. Each person has a different response to specific types of food, as a great diet for one...
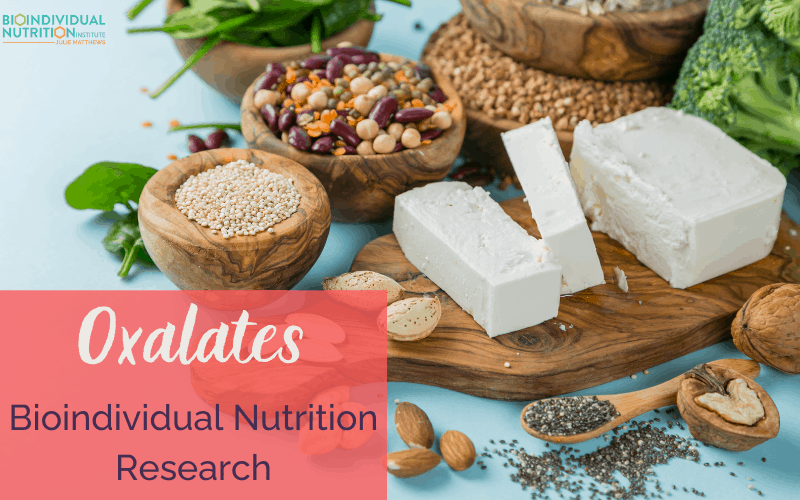
by Julie Matthews, MS | Sep 17, 2021 | All Posts, BioIndividual Nutrition Articles
In the past, oxalate research was relegated to kidney-related disorders. Now much more is known. Whether your interest is autism, cancer, chronic pain, autoimmunity, or generalized inflammation, understanding the role dietary oxalates can play is critical for your...







