
by Julie Matthews, MS | Jan 3, 2024 | BioIndividual Nutrition Articles, Chronic Conditions, Scientific Research
New science and clinical experience reveal concerns about oxalates that far exceed traditional kidney stone pathology. In order to best support their patients and clients, integrative practitioners, and especially diet and nutrition specialists would benefit from...

by Julie Matthews, MS | Aug 5, 2016 | Autism & Pediatric Articles, Chronic Conditions, Scientific Research
>> DOWNLOAD and read the PDF of this article In my earlier article about oxalates, I explained how oxalates influence the biochemical progression and symptomatic expression of varied chronic diseases. Through an overarching “lens” of 18 years’ research and...
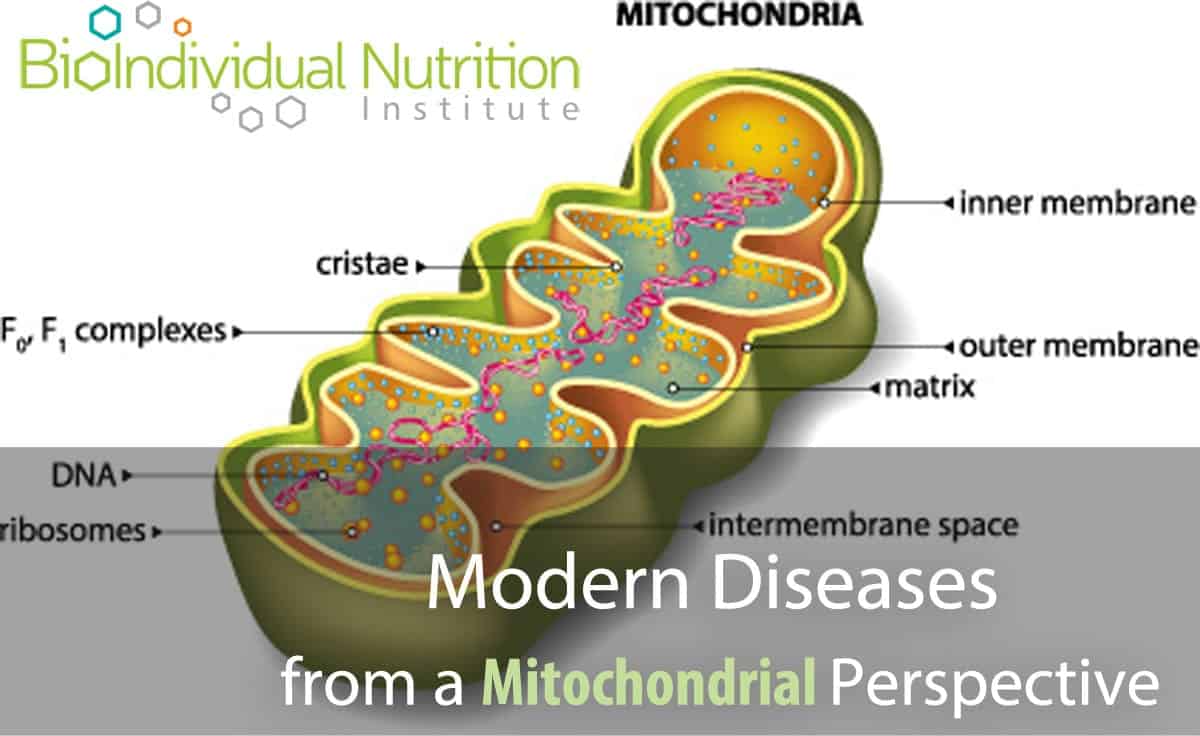
by Julie Matthews, MS | Jul 23, 2016 | BioIndividual Nutrition Articles, Chronic Conditions, Scientific Research
A growing body of research is implicating mitochondrial dysfunction as a root cause of a wide spectrum of metabolic, lifestyle and degenerative diseases including: Chronic Fatigue Syndrome, Autism, Cancer, Diabetes, Alzheimer’s, Fibromyalgia and potentially many more....




